Breathtaking Innovation
When lung infections are on the line, every minute counts.
The rapid Allora BreathTest™, currently in late-stage clinical development, can detect lung bacterial infection in 10 minutes.
When lung infections are on the line, every minute counts.
The rapid Allora BreathTest™, currently in late-stage clinical development, can detect lung bacterial infection in 10 minutes.
The only test that has the potential to differentiate a virulent bacterial infection from strep pneumonia and viral lung infection.
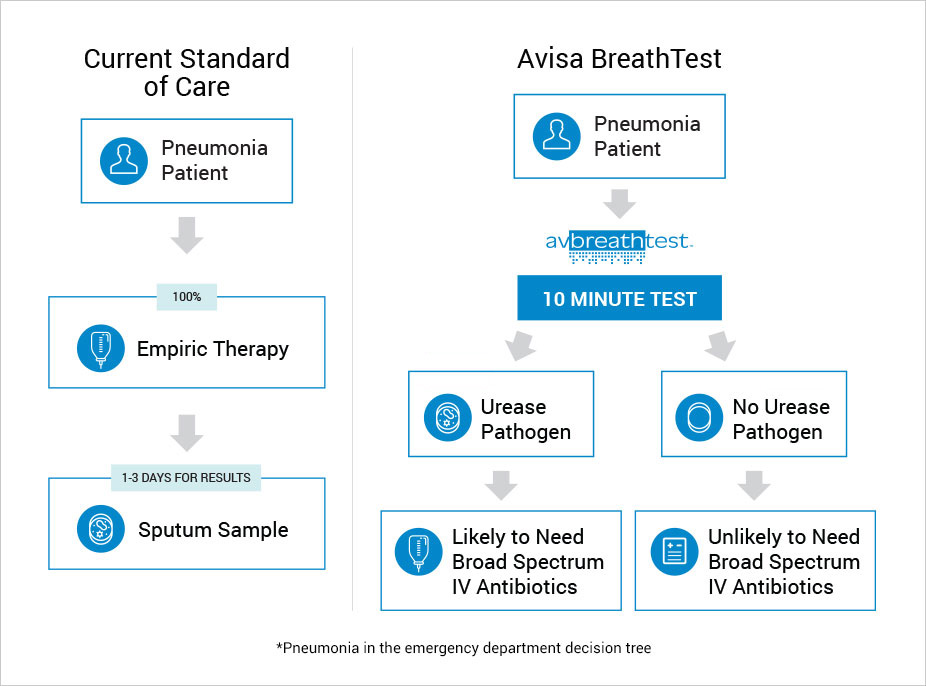
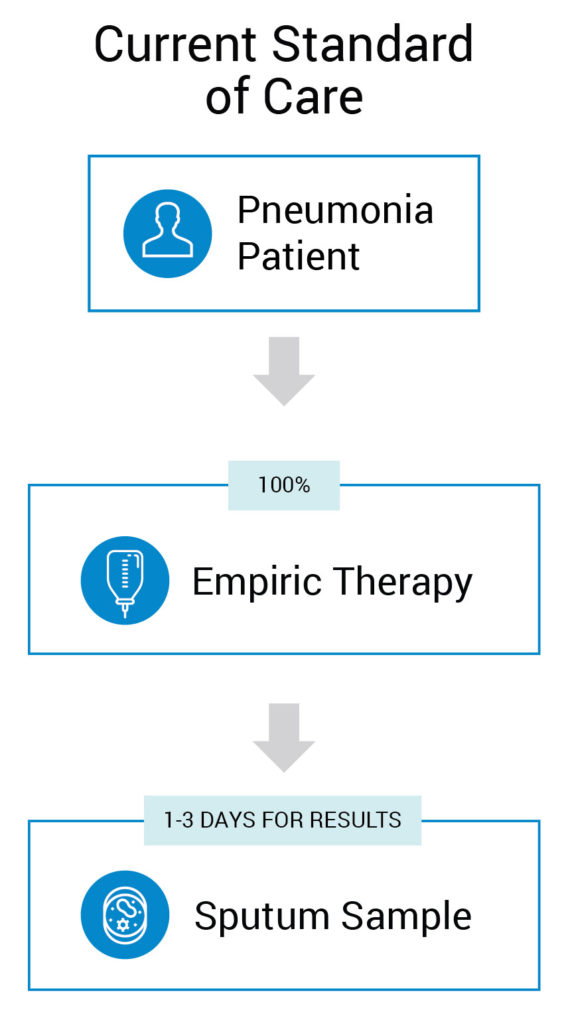
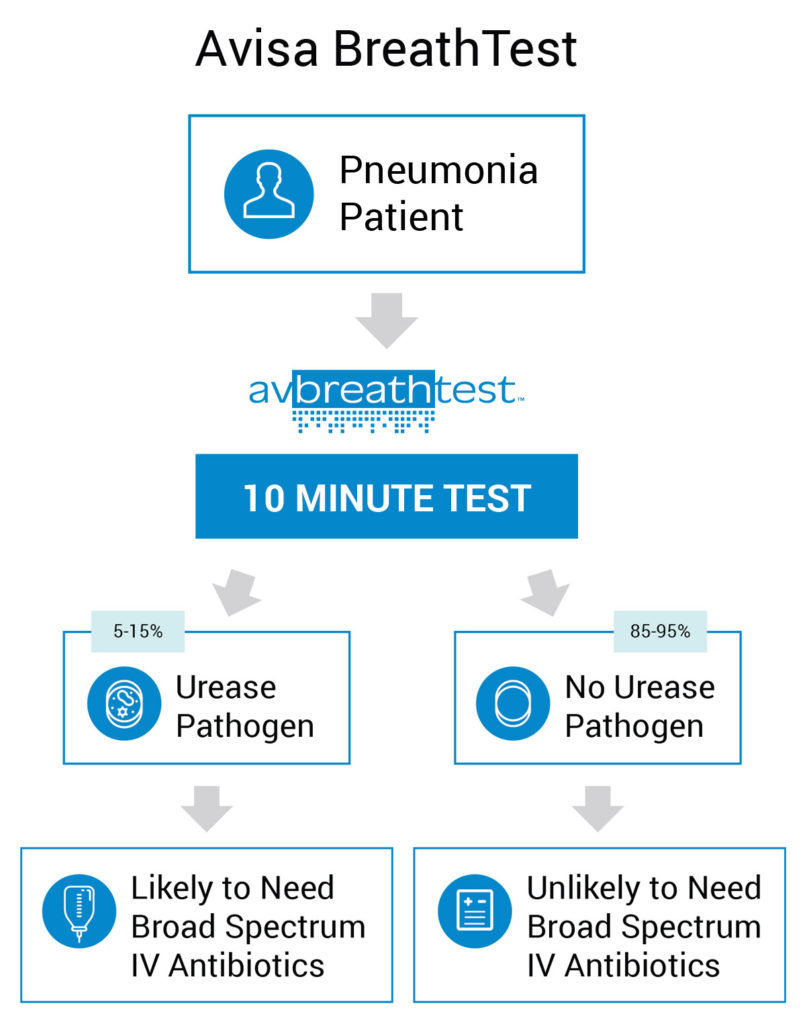
A Value-Based, Cost Advantaged Product would be a Win for Patient, Doctor, Hospital and Payer
| ABT Portfolio | ABT Indications | IDE/Pivotal | |
|---|---|---|---|
| Ventilator Associated Pneumonia | 2023 | ||
| Post-Covid 19 Bronchiectasis | Investigator Sponsored | ||
| Chronic Obstructive Pulmonary Disease (COPD) |
Investigator Sponsored | ||
| Community-Acquired Pneumonia (CAP) |
Investigator Sponsored | ||
| Cystic Fibrosis | Investigator Sponsored | ||
| Tuberculosis | Investigator Sponsored | ||
| Aspergillus/Coccii Fungus | Investigator Sponsored | ||
| Clostridium Difficile (C. diff) | Developmental |