A biomarker point-of-care test for rapidly detecting pulmonary infections
The Allora BreathTestTM is a biomarker, quantitative, point-of-care test for rapidly detecting pulmonary infections due to certain virulent pathogens without the need to collect and culture sputum or other biological samples.
How does it work?
Urease Converts 13C-urea to 13CO2
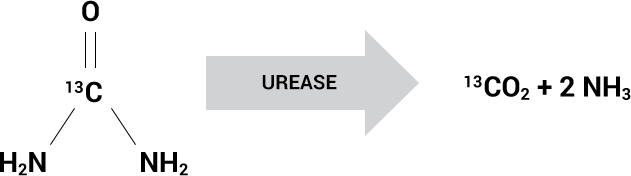
Based on the presence of the urease enzyme
The Allora BreathTestTM is based on the presence of the urease enzyme found in certain bacterial species that cause pneumonia, such as S. aureus, P. aeruginosa, Klebsiella and H. influenzae.
Live urease-containing bacteria can be detected using inhaled 13C-urea which is converted by these bacteria to labeled carbon dioxide (13CO2) and ammonia. The non-radioactive, isotopic ratio of 13CO2 to naturally occurring 12CO2 is measured in the exhaled breath of the patient and is akin to an infection thermometer for the lungs.
How is it different?
Results in less than 10 minutes
Patient inhales nebulized AV-U13, 13C-urea

Bacteria rapidly converts AV-U13, to 13CO2 that is exhaled

AVISAR Laser Spectrophotometer measures 13CO2

Point-of-care analysis in seconds
Quickly detects live urease pathogens anywhere in the lung within 10 minutes
The primary differentiators of the Allora BreathTestTM are its inherent ability to quickly detect live urease pathogens anywhere in the lung within 10 minutes; the use of a proprietary, nebulized 13C-urea (AV-U13) drug for inhalation; and the development of the AVISARTM, a point-of-care medical device that replaces much larger laboratory-based instrumentation typically used for measuring exhaled CO2.
Conveniently packaged in a disposable breath collection kit
The AV-U13 drug and nebulizer are conveniently packaged in a disposable breath collection kit. The Allora BreathTestTM reports the quantitative difference in the 13CO2 to 12CO2 ratio between baseline and post-nebulization breath samples in per mil units. Large differences in this ratio indicate an active pulmonary infection with a urease pathogen.
Allora BreathTestTM Kit
The Allora BreathTest kit is a single-use, disposable kit
containing the following items:
- Lyophilized AV-U13 drug
- 3 ml pre-filled syringe of sterile water
- Breath collection and biologic filter components
- Aerogen Solo Mesh Nebulizer






AVISARTM
Laser spectrometer with nebulizer
Allora is in the final stages of developing the AVISARTM instrument. The AVISARTM contains a touchscreen operator interface, nebulizer controller, breath sampling pneumatics and a laser spectrometer.
The AVISARTM rapidly measures breath 13CO2/12CO2 isotopic ratios using near infrared wavelength modulation spectroscopy enabled by a patented, tunable semiconductor laser.
Major design innovations have resulted in a compact, portable device weighing about 9 pounds (4 kg), 8.5” x 11” x 3.5” that is deployable in point-of-care settings such as clinics, hospital wards and intensive care units.
The Allora BreathTestTM is currently an investigational device and has not yet been approved by the FDA for commercial use.